Chemists from St Petersburg University propose an environmentally friendly method of synthesising rare earth metal derivatives using ultrasound
Scientists from St Petersburg University and Peter the Great St Petersburg Polytechnic University have developed an environmentally friendly method for the synthesis of lanthanides — rare earth metals used in materials science. The synthesis is carried out in the presence of ultrasound and, unlike classical approaches, does not require special temperature conditions, making it easily scalable for production purposes.
The research findings are published in the scientific journal Chemistry.
Rare earth metals (REMs) are a group of 17 chemical elements, 15 of which are lanthanides. Some of these compounds are even more abundant in the Earth’s crust than lead or gold, but they are difficult to mine and purify because their concentration in minerals is extremely low. Additionally, rare earth metals almost always occur together in ores, and their compounds are chemically very similar, making them particularly difficult to separate.
The lanthanides include lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, and yttrium.
Rare earth metals are a crucial component of modern microelectronics and are widely used in the nuclear and aerospace industries, as well as in the manufacture of catalysts and lasers. The extraction and high-quality processing of these elements is therefore a strategic priority for many countries.
Chemists from St Petersburg University have long been engaged in the synthesis and development of lanthanides to enhance their key properties, and previously proposed a method for producing hybrid luminescent polymers for use in sensors and gadget screens.
In their new research, the experts from St Petersburg University have developed a method for the synthesis of microcrystalline metal-organic frameworks of lanthanides, belonging to the group of rare earth metals.
Metal-organic frameworks (MOFs) are hybrid materials that combine the properties of inorganic and organic compounds. Their high porosity, tunable structure, and functionality offer prospects in catalysis, gas separation, sensing, and medicine. Particularly promising are lanthanide-based MOFs, which exhibit luminescence and have applications in light-emitting diodes, bioimaging, and contaminant detection. However, classical synthesis methods require high temperatures and long lead times, making them difficult to scale up.
‘We have developed an environmentally friendly method to synthesise fine materials using ultrasound. A solution of sodium terephthalate was added drop by drop to solutions of lanthanide salts placed in an ultrasonic bath. As a result of the reaction, microparticles of lanthanide terephthalates precipitated and were then easily separated by centrifugation. Unlike traditional methods, our proposed approach does not require the addition of surfactants or organic solvents, which makes the process more environmentally friendly,’ explained Andrey Mereshchenko, Head of the research team and Associate Professor in the Department of Laser Chemistry and Laser Materials Science at St Petersburg University.
The method is easily scalable and can be adapted for the preparation of other metal-organic compounds, opening up new opportunities in materials science. The materials obtained have a high specific surface area, making them promising for sorption and catalysis, and the luminescent properties of some compounds allow their use in sensors for the detection of heavy metals and organic compounds.
Due to their controlled morphology and structure, lanthanide terephthalates can be used in various fields, including environmental protection and photonics. In future, the scientists from St Petersburg University plan to investigate the luminescent and catalytic properties of such rare earth metal compounds in more detail to reveal their full potential and applications.
The project ‘Heterometallic terephthalates of rare-earth elements for luminescent sensors design’ is supported by a grant from the Russian Science Foundation. The research was carried out at the Department of Laser Chemistry and Laser Materials Science at St Petersburg University using the resources and infrastructure of the University’s Research Park.
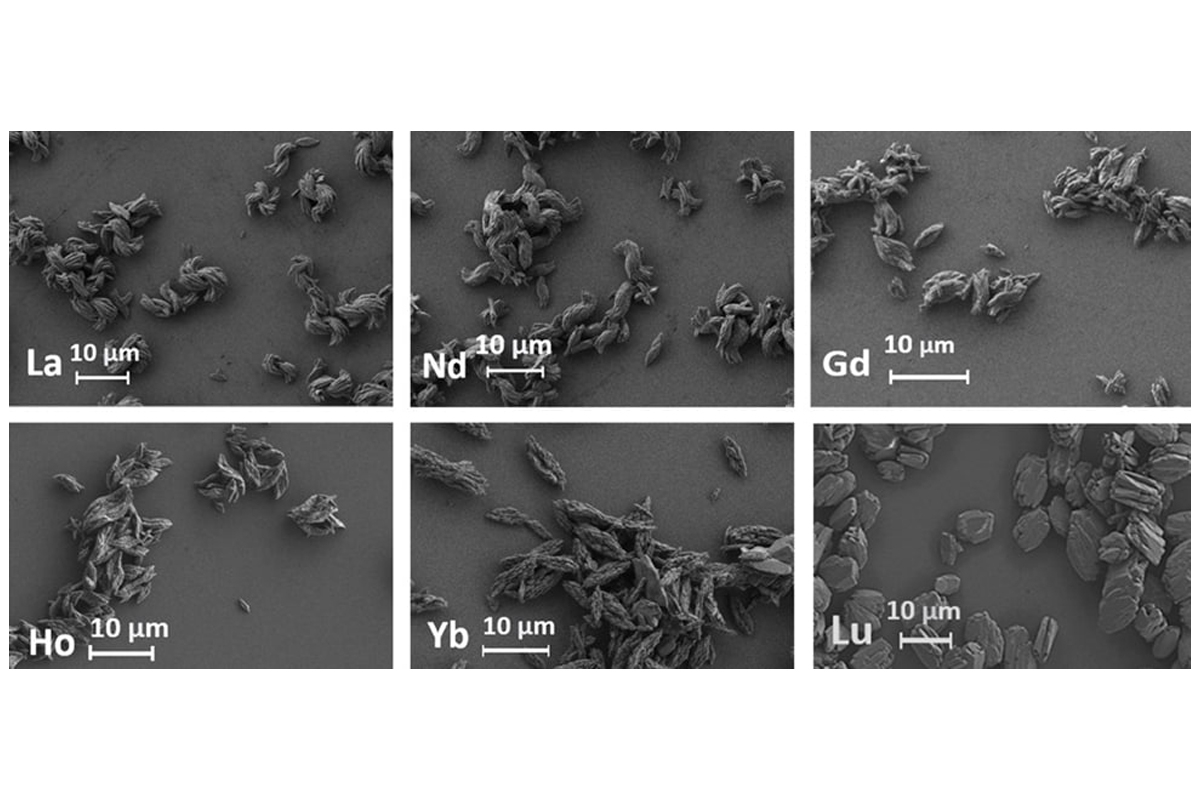
‘Electron microscopy studies have shown that the terephthalate particles of most lanthanides have the shape of oval plates, ranging in size from 2 to 10 µm. Lutetium terephthalate particles have the shape of microscopic bricks, confirming the influence of structure on crystal morphology. We also found that ultrasound treatment prevented particle aggregation, while synthesis without ultrasound led to the formation of large heterogeneous aggregates,’ commented Andrey Mereshchenko, Head of the research group and Associate Professor in the Department of Laser Chemistry and Laser Materials Science at St Petersburg University.
St Petersburg University, the oldest university in Russia, was founded on 28 January (8 February) 1724. This is the day when Peter the Great issued a decree establishing the University and the Russian Academy of Sciences. St Petersburg University today is a major centre for education and research. More than 20,000 students study here, and more than 15 major laboratories and 23 resource centres have been established as part of the country’s leading Research Park. Graduates of the University have been recipients of the Nobel and Fields Prizes on multiple occasions.
Recently, St Petersburg, the Northern Capital of Russia, officially introduced a new holiday—Day of St Petersburg University—which has been included in the St Petersburg Law ‘On holidays and commemorative days in St Petersburg’. In February 2025, a ceremonial event was held, during which Roscosmos cosmonauts presented the University with the ‘300th nniversary of St Petersburg University’ flag, which had travelled to the International Space Station and back.

