Scientists from St Petersburg University discover a new mechanism for the formation of nanocrystals for optoelectronic devices
Scientists from St Petersburg University have identified a new mechanism for the formation of core-shell nanowires, composed of indium, gallium, and nitrogen, with a high indium content in the core. The resulting nanostructures exhibit intense emission at room temperature, making them suitable for the development of advanced light-emitting diodes (LEDs) and lasers.
The InGaN (indium gallium nitride) alloy, used today in power electronics and LEDs, also shows promise for applications in gas sensors, solar cells, and hydrogen cells. However, its widespread use is constrained by challenges in synthesising a stable layer.
The research findings are published in the top-rated scientific journal Nanoscale Horizons.
Recently, scientists from St Petersburg University have conducted detailed studies on the mechanisms of formation of three-dimensional (non-planar) structures based on InGaN material, employing scientific and systematic approaches to describe their growth processes. Using such compounds, St Petersburg University is already developing prototypes of LEDs, gas sensors, cells for water decomposition, and more.
As the physicists explained, in the conventional "planar" form of the scientific approach, complex microelectronic structures are created on a flat surface through several successive steps, including material deposition, etching, and lithography, to form various layers and components of a device. However, in the case of InGaN, forming such planar structures using traditional methods is not feasible.
The miscibility gap effect makes it challenging to obtain InGaN layers with high indium (In) content, as this often results in material decomposition into separate phases and the formation of numerous defects. Furthermore, mismatches in the lattice constants of these materials also contribute to the formation of defects. Together, these factors significantly reduce the operational performance of devices that incorporate such structures.
The physicists from St Petersburg University have discovered a novel mechanism for the formation of nanocrystals based on InGaN material directly on a silicon surface.
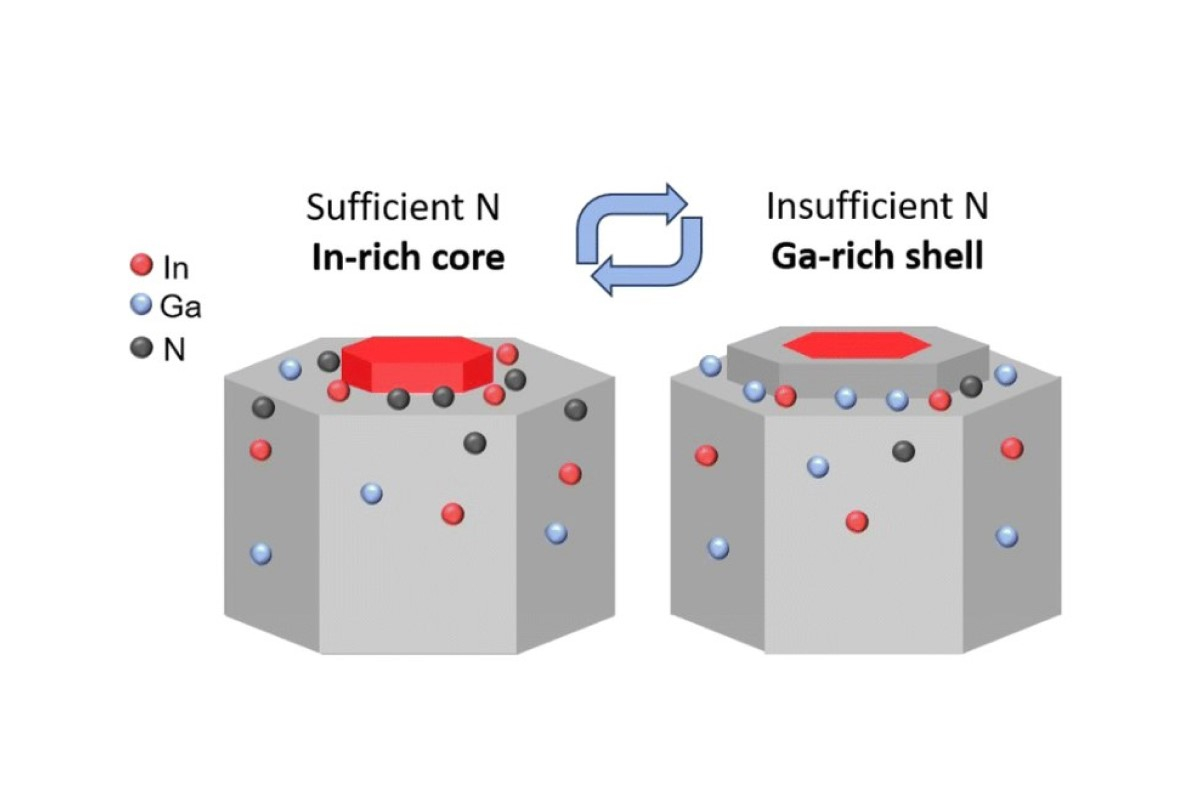
In particular, we have explained, for the first time, a new mechanism for the formation of spontaneous core-shell lnGaN nanowires. Experimental results demonstrated that the indium content in the core of the nanocrystal can reach approximately 40% or higher, while in the shell, it is about 4%.
Rodion Reznik, the author of the development, Head of the Laboratory of New Semiconductor Materials for Quantum Informatics and Telecommunications at St Petersburg University
"It is important to note that achieving such a high indium content in high-quality InGaN layers is extremely challenging, but we have managed to do it," said Rodion Reznik, the author of the development, Head of the Laboratory of New Semiconductor Materials for Quantum Informatics and Telecommunications at St Petersburg University.
Increasing the indium content in InGaN alters the wavelength — effectively changing the colour of the emission — of these nanostructures. This significantly broadens the potential applications of this material in the development of new, highly efficient LEDs, lasers, solar cells, and more. The intense emission observed from the nanostructures created by the scientists highlights the high optical quality of the new material.
"The results of theoretical studies have, for the first time, revealed that the formation of core-shell heterostructures in non-catalytic InGaN nanowires is linked to periodic changes in growth conditions at the top of these nanostructures. Even during the growth of a single monolayer of such a nanostructure, the ratio of group III and group V atoms from the periodic table at the top of the structure changes," explained Rodion Reznik, Head of the Laboratory of New Semiconductor Materials for Quantum Informatics and Telecommunications at St Petersburg University.
According to Rodion Reznik, during the initial stage of nanowire growth, balanced conditions allow the miscibility gap effect to be overcome, enabling the formation of a nanowire core enriched with indium. Subsequently, the conditions shift to become group III-enriched, altering the mechanism of shell formation. Notably, the shell can be effectively removed by chemical methods without compromising the quality of the core.
For reference: the staff of the Laboratory of New Semiconductor Materials for Quantum Informatics and Telecommunications at St Petersburg University are studying new materials for microelectronics: single photon sources, efficient LEDs, solar cells, lasers, nano piezo generators, and their integration with the silicon platform. All these achievements continue the work of two Nobel laureates to improve quantum technologies for microelectronics: Alexey Ekimov, Nobel Laureate in Chemistry and a graduate of St Petersburg University; and Zhores Alferov, the organiser and Rector of Saint Petersburg National Research Academic University. Rodion Reznik talked more about his work on St Petersburg University’s Heinrich Terahertz podcast.
St Petersburg University, the oldest university in Russia, was founded on 28 January (8 February) 1724. This is the day when Peter the Great issued a decree establishing the University and the Russian Academy of Sciences. Today, St Petersburg University is an internationally recognised centre for education, research and culture. In 2024, St Petersburg University celebrates its 300th anniversary.
The plan of events during the celebration of the anniversary of the University was approved at the meeting of the Organising Committee for the celebration of St Petersburg University’s 300th anniversary. The meeting was chaired by Dmitry Chernyshenko, Deputy Prime Minister of the Russian Federation. Among the events are: the naming of a minor planet in honour of St Petersburg University; the issuance of bank cards with a special design; and the branding of the aircraft of the Rossiya Airlines to name just a few. To mark the 300th anniversary of St Petersburg University, a postage stamp depicting the Twelve Collegia building and the monument to Count Sergey Uvarov was issued. Also, a Soyuz rocket bearing the symbols of the University was launched from the Baikonur Cosmodrome.
By the decision of the Governor of St Petersburg Alexander Beglov, 2024 is a year of the 300th anniversary of St Petersburg University in St Petersburg. On the day of the University’s 300th anniversary torches were lit on the Rostral Columns on the Spit of Vasilyevsky Island. St Petersburg University flags were raised on the Palace Bridge. The city public transport was decorated with the University’s symbols. During St Petersburg’s City Day celebrations in May 2024, St Petersburg University acted as a participating venue. Additionally, the University has launched a website dedicated to the anniversary. The website contains information about outstanding University staff, students, and alumni; scientific achievements; and details of events held as part of the celebration of the 300th anniversary of the University.

