Chemists from St Petersburg University and the Kola Science Centre at the Russian Academy of Sciences develop a method for recycling thermal power plant fly ash to produce geopolymers with enhanced strength
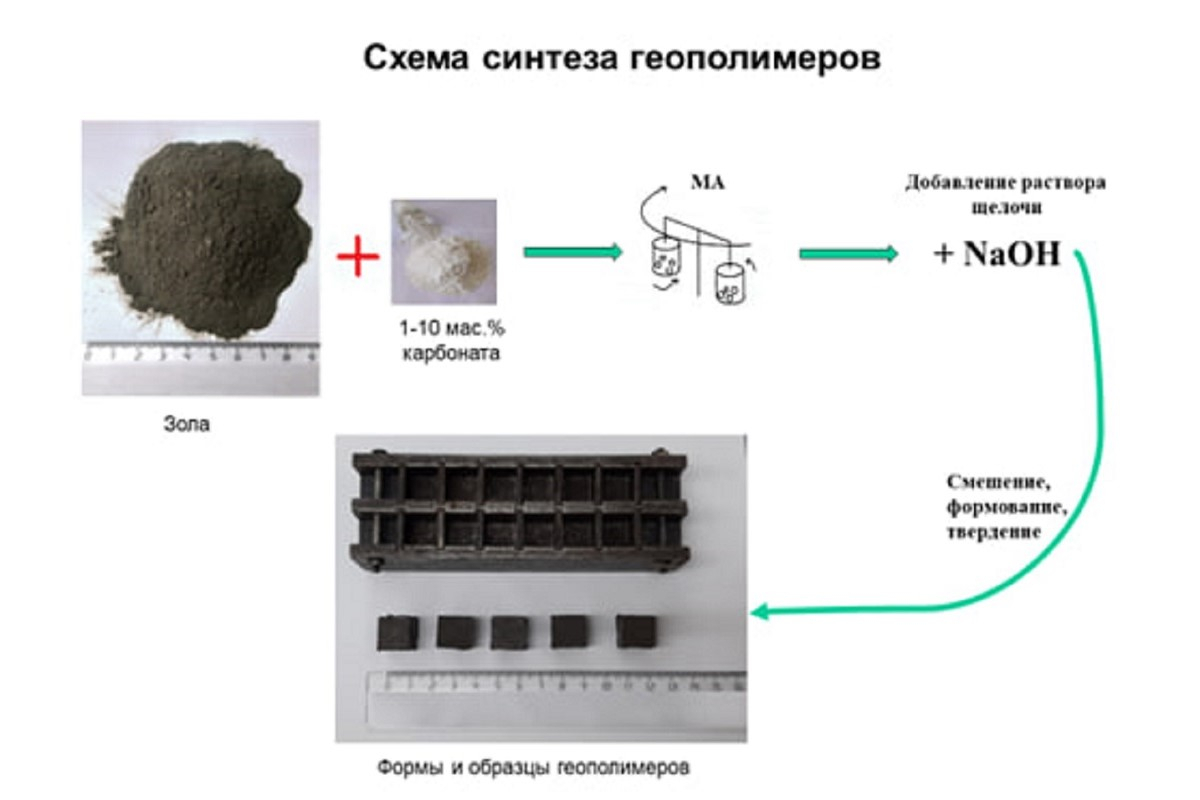
Scientists have synthesised geopolymers based on mechanically activated mixtures of fly ash with the carbonate mineral (СаСО3). These geopolymers can find application within the construction industry. The research team includes scientists from St Petersburg University and the Tananaev Institute of Chemistry and Technology of Rare Elements and Mineral Raw Materials at the Kola Science Centre of the Russian Academy of Sciences. Today, it is one of the most environmentally friendly methods for thermal power plant waste disposal and management.
In Russia, coal energy industry annually produces about 22 million tons of fly ash and slag. Global coal energy industry produces 800 million tons annually. In Russia, industrial solid by-products from coal-fired thermal power plants are estimated at 1.5 to 2 billion tons, and they occupy an area of more than 20,000 square km.
The research findings are published in the scientific journal Minerals.
Among the methods of utilisation of fly ash and other industrial solid by-products is to convert these industrial residues into safe and value-added materials, i.e. building materials used in construction. High-calcium fly ash and slag can be used in cement production, while low-calcium fly ash and slag can be used as fillers in concrete mixtures. Yet, currently no more than 10% of waste is subject to such disposal. One of the reasons is the rigidity of our market. Another factor is myths among consumers about the non-environmental friendliness and even harm of the product. This is far from being true. Technologies for fly ash recycling are successfully used across the globe. In Europe, 98% of fly ash and slag is utilised, in Japan — 96%, and in China — 80%. Yet there are no limits to what science can explore and scientists are looking for new, more eco-friendly methods for fly ash utilisation.
In recent years, scientists have been working on a technology for using low-calcium fly ash for the synthesis of geopolymer materials. Such materials are obtained by the interaction of natural and man-made aluminosilicate raw materials, including thermal power plant fly ash, with an alkaline agent, i.e. sodium hydroxide solution or liquid glass. Geopolymers are considered as an environmentally more beneficial alternative compared to traditional cement, since they can act as strong and durable construction materials, for example, cement and concrete. Geopolymers have a set of valuable physical and chemical properties. Owing to these properties, geopolymers find application in diverse fields such as refractories, fire-resistant materials, wastewater treatment, and radiation shielding.
Yet, this method has a drawback, say chemists. Not all classes of fly ashes, when interacting with an alkaline agent, produce materials of the required quality. To address this problem, researchers use mechanical activation or milling the fly ash in mills or introduce various additives to the fly ash. The chemical effect is the least studied one due to the complicated nature of geopolymerisation reactions.
The scientists from St Petersburg University and the Tananaev Institute of Chemistry and Technology of Rare Elements and Mineral Raw Materials were the first to combine the beneficial impact of adding carbonates to fly ash and that of mechanical activation.
'Our research has shown that by mixing the fly ash with the carbonate CaCO3 (calcite) as an additive we can observe a so-called synergistic effect. In other words, our study demonstrated that incorporating calcite into the fly ash, followed by mechanical activation, notably enhanced the compressive strength of geopolymers, unlike if applied separately,' said Irina Zvereva, the author of the study, Professor in the Department of Chemical Thermodynamics and Kinetics at St Petersburg University. This can be compared to how in ancient times people began to combine clay and reeds when building houses, she said. Reed-reinforced clay walls were much stronger and more effective than walls built only from clay or only from reed.
To gain a deeper insight into this process, the scientists also studied the influence of introducing to the fly ash of carbonates of other metals, i.e. magnesium, strontium and barium that are neighbours of calcium in the Periodic Table of Dmitri Mendeleev, a famous University scientist, whose 190th birthday is celebrated in 2024.
Dmitri Mendeleev was born on 8 February 1834. He is an outstanding scientist who built up the Periodic Table, the author of the fundamental works in chemistry, physics, geology, metrology, and other sciences. He lived and worked at the University. Today, his office is the Dmitri Mendeleev Museum and Archives at St Petersburg University.
Thermochemical studies at St Petersburg University and subsequent experiments showed that the ability of carbonate to react with an alkaline agent, i.e. sodium hydroxide solution, plays an important role. Magnesium carbonate interacts with alkali most actively, while strontium and barium carbonates are practically inert. Calcite occupies an intermediate position, which provides geopolymers with the greatest efficiency.
'Joint mechanical activation of the fly ash and calcite in a mill does not simply lead to their mixing and a reduction in particle sizes. When a particle is split, the chemical bonds that hold it together are broken, revealing a fresh surface rich in active sites. As a result, the fly ash becomes more reactive and dissolves more intensively in the alkali. Compared to the fly ash, calcite, which is more resistant to alkali, also increases its activity after mechanical treatment together with the fly ash in a mill. Mechanically activated calcite partially dissolves in alkali and at the same time is to some extent transformed into two newly formed substances, i.e. lime (calcium hydroxide) and vaterite. It is known that the surfaces of newly formed substances are also rich in active centres. All these centres, those induced mechanochemically in calcite and «newborns» in lime and vaterite, play an important role in the synergistic effect,' said Professor Aleksandr Kalinkin, Head of the Department of Technology of Silicate Materials at the Tananaev Institute of Chemistry and Technology of Rare Elements and Mineral Raw Materials.
Calcite is one of the most accessible minerals, found everywhere in the form of limestone, chalk, marble, and shell rocks, which cover tens of millions of square km of the Earth’s surface. Its use as part of geopolymers can easily and cheaply improve the environmental situation at thermal power plans, allowing not only utilisation of the fly ash and other industrial solid by-products, but also converting industrial residues into safe and value-added materials.
The article shows that strontium and barium carbonates, although they do not significantly increase the strength of geopolymers like calcium carbonate, are reliably embedded in the geopolymer matrix. The geopolymers based on the fly ash blended with carbonates exhibit potential applications in immobilising radioactive strontium. Barium carbonate absorbs X-rays and gamma rays, so geopolymers based on barium carbonate can be used for producing radiation shielding materials.
St Petersburg University, the oldest university in Russia, was founded on 28 January (8 February) 1724. This is the day when Peter the Great issued a decree establishing the University and the Russian Academy of Sciences. Today, St Petersburg University is an internationally recognised centre for education, research and culture. In 2024, St Petersburg University will celebrate its 300th anniversary.
The plan of events during the celebration of the anniversary of the University was approved at the meeting of the Organising Committee for the celebration of St Petersburg University’s 300th anniversary. The meeting was chaired by Dmitry Chernyshenko, Deputy Prime Minister of the Russian Federation. Among the events are: the naming of a minor planet in honour of St Petersburg University; the issuance of bank cards with a special design; the creation of postage stamps dedicated to the history of the oldest university in Russia; and the branding of the aircraft of the Rossiya Airlines to name just a few.
By the decision of the Governor of St Petersburg Alexander Beglov, 2024 is a year of the 300th anniversary of St Petersburg University in St Petersburg. On the day of the University’s 300th anniversary torches will be lit on the Rostral Columns on the Spit of Vasilyevsky Island. St Petersburg University flags will be raised on the Palace Bridge. The city public transport will be decorated with the University’s symbols. New tourist maps will feature the locations of the University buildings, with thematic and historical materials about the University placed nearby. During St Petersburg’s City Day celebrations in May 2024, St Petersburg University will be a participating venue. The traditional "Scarlet Sails Festival" will also be dedicated to the anniversaries of St Petersburg University and the Russian Academy of Sciences. Additionally, the University has launched a website dedicated to the upcoming holiday. The website contains information about outstanding University staff, students, and alumni; scientific achievements; and details of preparations for the anniversary.

