St Petersburg University chemists find a speedy and efficient way to recycle the graphite anodes of Li-ion batteries
St Petersburg University scientists have developed an energy-efficient and fast method to recycle graphite anodes, one of the main elements of lithium-ion batteries.
Today, Li-ion batteries, or LIB, are used in: home appliances; cell phones; laptops; digital cameras; camcorders; and electric cars. The recycling of LIB is becoming increasingly important both in Russia and worldwide. The growing interest in exploring methods of such recycling is caused by: the desire to reduce environmental impact; the possibility of valuable metals recovery; and the opportunity of restoring the working capacity of cathode and anode materials. On the one hand, that will significantly reduce the harmful impact on the environment. On the other hand, that will make it possible to reuse materials, and therefore reduce equipment production costs. So, scientists are actively exploring potential methods to improve the efficiency of such batteries and ways to recycle them.
The research findings are published in the Journal of Environmental Chemical Engineering.
Nowadays, graphite is widely used in the production of LIB. The difficulty of recycling this carbon structure lies in the fact that an unstable and inhomogeneous solid electrolyte layer is often formed on the recycled graphite. That leads to the destruction of the graphite structure and its accelerated ageing. St Petersburg University scientists have proposed a new method for recycling graphite anodes. It will make it possible not only to clean it faster, but also to form graphene oxide from the surface graphite layers. Graphene oxide is a conductive and degradation-resistant structure.
’The method proposed by us is notable for its simplicity. It is an economically advantageous one-stage method of recycling spent graphite anodes using a plasma discharge over the surface of a liquid when graphite is dispersed (powdered),’ said Evgenii Beletskii, a research associate in the Department of Electrochemistry at St Petersburg University.

During the recycling, the graphite coating is first separated from the copper conductor. For that, the graphite sheets are simply mixed in distilled water. The reaction of intercalated lithium with water results in abundant gassing and separation of the coating from the copper. The resulting dispersion is washed, centrifuged, transferred to a reactor, and enriched with a hydrogen peroxide solution. Then, with constant stirring, it is treated with an up to 1,000 V electrical discharge in vacuum. A glow discharge occurs between the working electrode and the dispersion surface. It is accompanied by UV and shock waves. Under their action, gradual decomposition of hydrogen peroxide takes place and active particles such as hydroxyl radicals are formed. They interact with the surface of dispersed particles, causing oxidation of both these particles and the electrolyte decomposition products on them. The shock waves help separate the oxidation products from the particles. As a result, surface-modified graphite is obtained. It retains its internal structure which ensures the preservation of the graphite capacity provided for by the manufacturer, plus a certain increase thereof due to surface modifiers.
The team of scientists used the infrastructure of the St Petersburg University Research Park, namely the equipment of the following resource centres: the Interdisciplinary Resource Centre for Nanotechnology; the Centre for Optical and Laser Materials Research; the Centre for Physical Methods of Surface Investigation; and the Centre for X-Ray Diffraction Studies. The research was supported by a scholarship of the President of the Russian Federation No SP-1045.2022.1. "Plasma electrochemical recycling of spent electrode materials of lithium-ion batteries for reuse in energy storage devices".
When this method is used, the energy consumption reaches 6.9 to 28 Wh per 1 kg of graphite. That is several hundred times less than in traditional recycling methods such as pyrometallurgy and hydrometallurgy. Using the latter ones, it is still impossible to recover anodic graphite. At the same time, the recycling period is also significantly less − only 30 to 60 minutes.
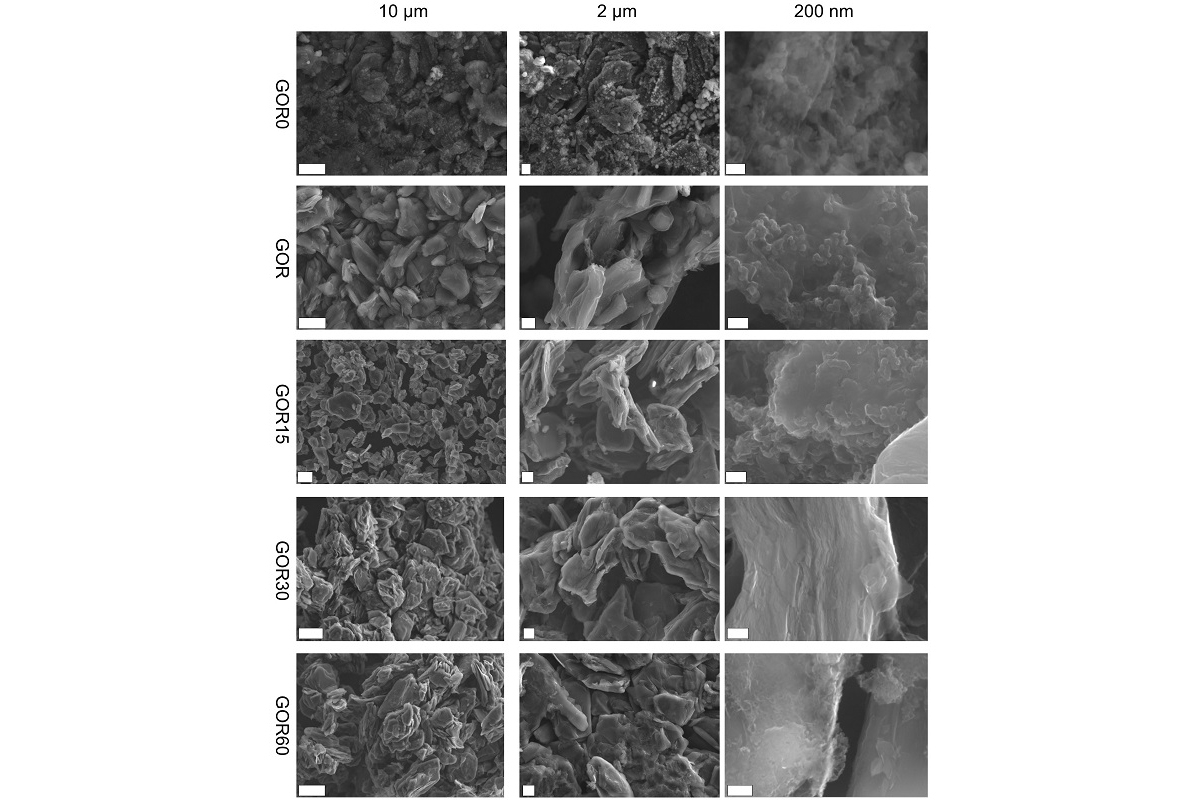
This result was achieved due to certain features the electrical discharge demonstrates in the liquid. When an electrical discharge is directed into the hydrogen peroxide solution in use, hydroxyl radicals − OH − are formed. They have a high oxidative potential due to the decomposition of hydrogen peroxide. By finding the optimal conditions for recycling graphite this way, it is possible to remove various additives and electrolyte decomposition products from it. That will make it possible to achieve the best electrochemical characteristics of the recycled material surpassing the initial graphite.

