Chemists from St Petersburg University and Tomsk State University Find a "Key" to the Ion "Lock"
Scientists from St Petersburg State University and Tomsk Polytechnic University have applied the 19th-century "lock and key" concept to complex inorganic molecules — polyoxometalates (POMs) — and discovered that iodonium cation compounds can serve as a "key" for them, triggering selective interactions between ions. This discovery paves the way for creating materials with predetermined properties and novel catalysts.
The interaction between enzymes and substrates in chemistry was described by German scientist Emil Fischer in 1894. Fischer observed that enzymes (biological catalysts) interact with their substrates (molecules they act upon) in a highly specific manner. He likened this interaction to a key that fits only a particular lock.
This analogy helped explain why enzymes are so selective in their work. Just as a key must have the correct shape to open a lock, a substrate molecule must precisely match the enzyme’s active site for a chemical reaction to occur.
The "lock and key" concept is still used by chemists today. Moreover, it has evolved and found application in various fields of chemistry, including inorganic chemistry. Scientists from St Petersburg State University and Tomsk Polytechnic University applied the "lock and key" principle to complex inorganic molecules — polyoxometalates (POMs). POMs are large anionic clusters consisting of oxygen atoms and transition metals such as molybdenum or tungsten. They possess unique properties and find applications in catalysis, materials science, and even medicine.
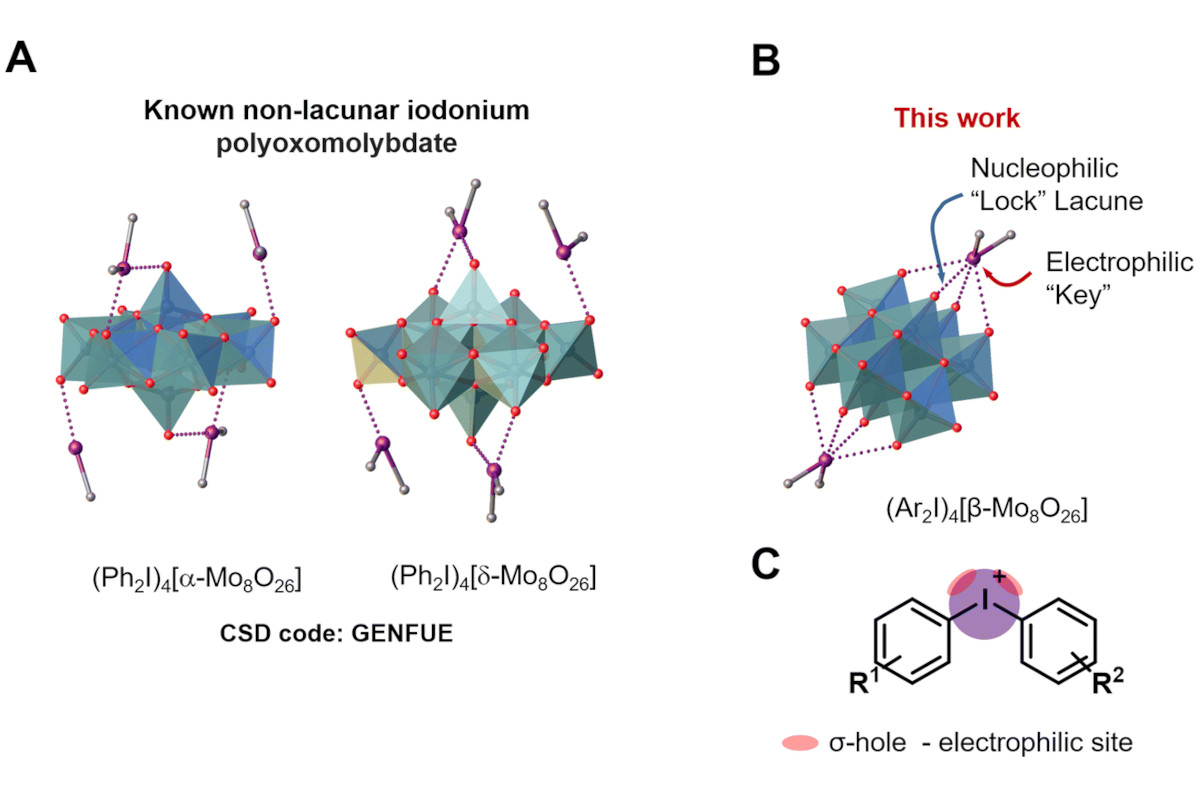
"During the study, the scientists discovered that a certain type of POM — the [β-Mo₈O₂₆]⁴⁻ anion — exhibits selectivity in its interaction with iodonium cations. This interaction can be described using the 'lock and key' analogy. In this case, the iodonium cation acts as the 'key', while the POM anion serves as the 'lock'. Interestingly, this selectivity is only observed for one isomer (type) of POM," explained one of the key authors of the study, Professor Dr. Vadim Kukushkin.
According to him, this interaction is due to the formation of a special type of intermolecular interaction between the cation and anion — halogen bonds. Halogen bonds have been attracting increasing attention from researchers in recent years due to their unique properties and potential applications in creating new materials.
The scientists note that this research demonstrates how fundamental principles, such as the ’lock and key’ concept, can find unexpected applications in modern chemistry. In the future, this discovery offers new possibilities for creating materials with predetermined properties based on specific interactions between ions. For example, such systems could be used to develop new types of sensors capable of selectively recognising certain ions in solution.
Another important horizon opened by this work is the development of new catalysts. POMs are already used in catalysis, but the ability to precisely control their interaction with other molecules opens the way for creating even more efficient and selective catalysts.
This work was made possible thanks to the collaboration of specialists from various fields at St Petersburg State University and Tomsk State University within the framework of the Mendeleev scientific and educational cluster. St Petersburg State University acts as the coordinator, assisting in the development and implementation of projects that require combining the potential and resources of the Mendeleev cluster member organisations.
"Similar to the 'lock and key' principle in the molecular world, the collaboration between Tomsk Polytechnic University and St Petersburg State University demonstrates 'complementarity' in science. TPU, with its technical expertise, acts as the 'key', while SPbU, with its fundamental base, serves as the 'lock'. Their interaction, like a perfect molecular match, creates synergy, opening new perspectives in the study of chemical processes and the creation of innovative materials," noted Professor Dr. Kukushkin.
The collaboration between chemists from TPU and SPbU has been ongoing for over 4 years. During this time, they have published more than 20 joint papers in authoritative international journals. The synergistic effect of their interaction has contributed to the successful implementation of seven Russian Science Foundation projects, a grant from the President of the Russian Federation, and a mega-grant from the Government of the Russian Federation.
Furthermore, the chemists collaborate in the educational component, actively exchanging students and postgraduates, organising staff internships, and conducting mutual visits of administrative representatives.
The collaboration has also resulted in the conference "Non-covalent Interactions in Synthesis, Catalysis, and Crystal Engineering", organised by representatives of SPbU and TPU in conjunction with the country’s leading scientific organisations.

