Scientists from St Petersburg University synthesise an analogue of the Earth’s most complex mineral in a laboratory
A team of scientists led by crystallographers from St Petersburg University has succeeded in synthesising an analogue of the Earth’s most structurally complex mineral, ewingite, in a laboratory.
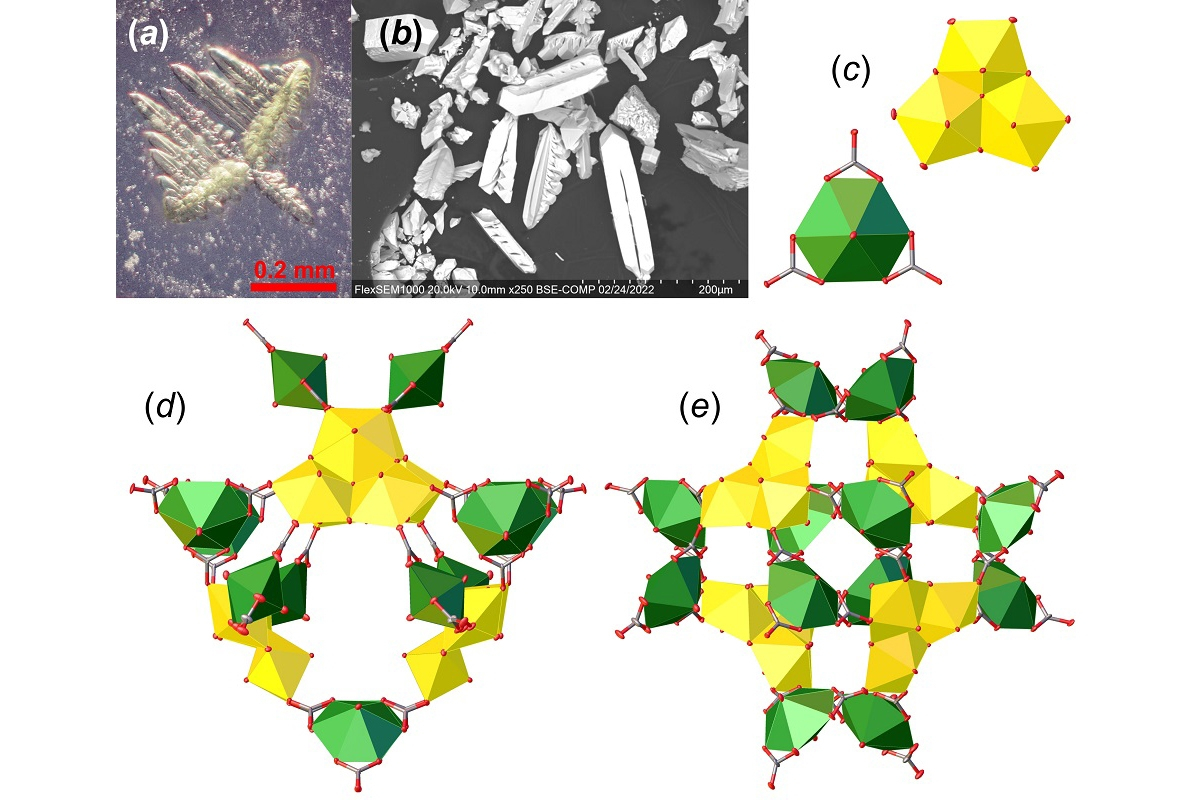
Ewingite is a mineral that was discovered in the mid-2010s in the abandoned Plavno uranium mine located in the Czech Republic. It is the most complex mineral known to exist on Earth. Moreover, because of the specific thermodynamic conditions required for its formation, the mineral is considered to be very rare.
The findings of the research supported by a grant from the Russian Science Foundation are published in the scientific journal Materials.
The researchers managed to synthesise an analogue with a composition and crystal structure similar to that of natural ewingite through a combination of low-temperature hydrothermal synthesis and room-temperature evaporation.
As the scientists pointed out, the stability of the mineral found is slightly higher than that of the synthetic compound (according to visual observations and experimental studies). However, the synthetic phase can be regarded as a primary and metastable reaction product which further re-crystallises into a more stable form under environmental conditions.
Vladislav Gurzhiy is Associate Professor in the Department of Crystallography at St Petersburg University, Chairman of the Scientific Committee of the Institute of Earth Sciences and Principal Investigator of the research. According to him, the uniqueness of ewingite lies in its crystal structure, which is based on nanoclusters formed by uranium atoms and carbonate groups. Such structural complexes were not known before the discovery of the mineral, either in nature or among synthetic compounds. The St Petersburg Universities scientists therefore wanted to synthesise this unique creation of nature in the laboratory.
‘Our team has quite a lot of experience in obtaining mineral analogues in the laboratory and synthesising new compounds with mineral-like structures. However, even with this experience, we worked on the experiment for almost a year and a half. This is how long it took to establish a synthesis protocol to produce large enough crystals to make it possible to obtain a reliable structural model,’ Vladislav Gurzhiy emphasised.
The compound obtained by the researchers in the laboratory differs slightly in composition from the natural one. In the structure of the mineral, the uranyl carbonate clusters are bonded through magnesium and calcium atoms, while the synthetic compound contains only calcium. However, this has no effect on the nanocluster packing principle in the crystal structure.
The research is supported by the grant from the Russian Science Foundation (project № 19-17-00038) and the Czech Science Foundation. X-ray phase and spectroscopic studies were performed at the Centre for X-ray Diffraction Studies of the St Petersburg University Research Park.
In addition to its fundamental scientific interest, this study is also of quite practical value, because even in depleted deposits like the Plavno mine, a significant amount of uranium remains in scattered form.
‘By understanding the mechanism of secondary mineral formation (recrystallisation), which results in the formation of ewingite, it will be possible to control the processes of uranium removal into the environment. Or, on the contrary, this will make it possible to create conditions under which, if necessary, it will be possible to convert uranium into a dissolved form so that it becomes less dangerous to humans,’ added Vladislav Gurzhiy.

