Indicator: Scientists discover Rubik's chemical snake that can be used to ‘fold’ new heterocyclic molecules
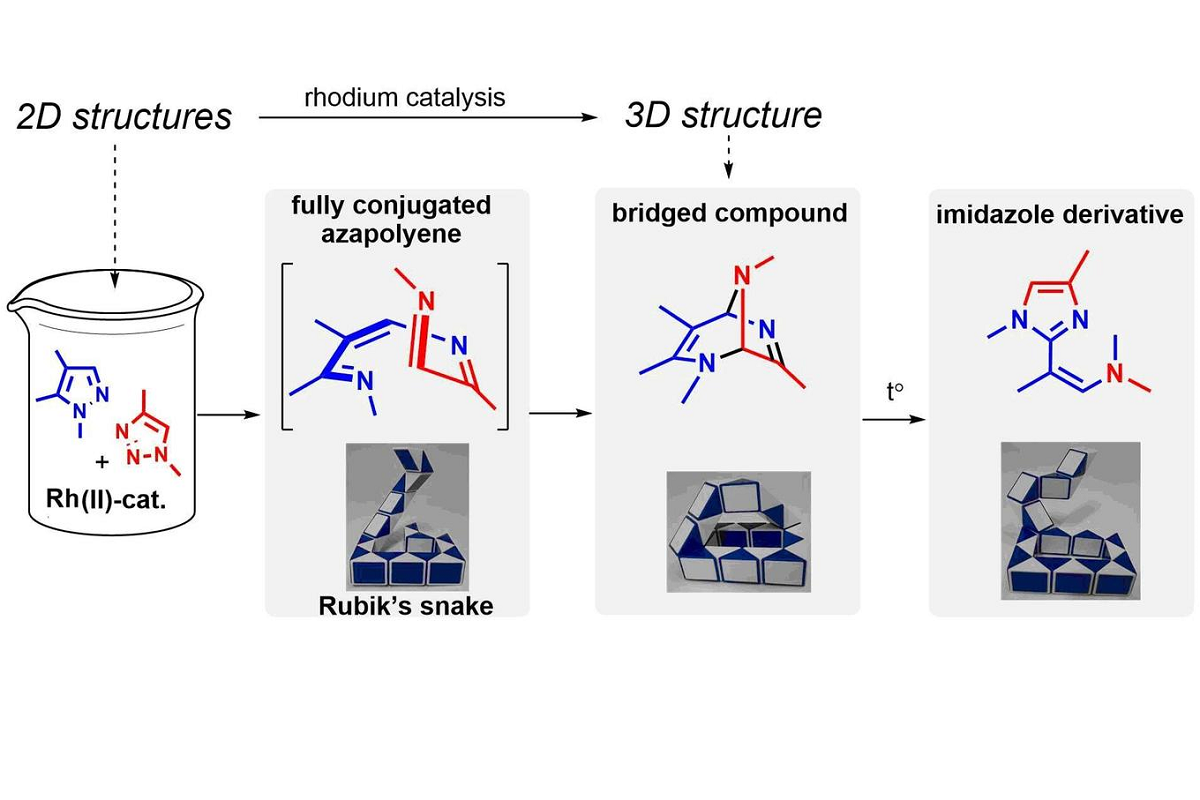
Chemists at St Petersburg University have discovered a new type of reaction that can synthesise complex polycyclic organic molecules. The source are unsaturated compounds. They have double or triple inter-carbon bonds whose structure resembles a Rubik's snake. In just one step, this reaction made it possible for the scientists to turn readily available and widely used in chemistry 2D heteroaromatic molecules into exotic and previously unknown 3D bridging structures.
The research was supported by a grant from the Russian Science Foundation. The research findings are published in the journal Organic Letters.
Pericyclic reactions in organic chemistry allow complex cyclic and polycyclic molecules to be synthesised from simpler ones in just one step, with all atoms remaining intact. The research group from St Petersburg University showed that conjugated azapolyenes, organic compounds with alternating single and double carbon-carbon bonds, in which some carbon atoms are replaced by nitrogen atoms, are very active in such reactions. In this case, the nitrogen-containing polyene molecule, unlike its fully carbon analogue, becomes similar to the well-known puzzle – a Rubik's snake, from which the most bizarre 2D and 3D constructions can be assembled.
The researchers have found a simple way to chemically obtain such nitrogen-containing polyenes from simple and accessible heteroaromatic compounds: pyrazoles and triazoles. The ‘Rubik's snakes’ synthesised in this way spontaneously ‘stack’ into highly unsaturated (having several double bonds) bridging bicyclic structures.
These compounds resemble alkaloids in structure, so they could potentially have biological activity and be used in medicinal chemistry.
Additionally, it turned out that even more complex cyclic molecules, which have boron and zinc atoms in their composition, could be synthesised on their basis.
‘We have discovered a process of self-assembly of fully conjugated nitrogen-containing polyenes. This produces highly unsaturated bridging bicyclic structures. This is obviously only the beginning of the amazing chemistry of these molecules. In subsequent experiments, we showed that by varying the heteroatoms, i.e. nitrogen and oxygen atoms as well as substituents in azapolyene, one can ‘stack’ it not only in a bi- but also in a tricyclic bridging structure. Unfortunately, such molecules are unstable. However, we suggest that their stability could be achieved by slightly lengthening the carbon chain of the original azapolyene. There is no doubt that the chemical Rubik's snake will surprise us with its ability to form a world of molecules with amazing structures,’ says Mikhail Novikov, Head of the project, Doctor of Chemistry, Professor at the Institute of Chemistry of St Petersburg University, Head of the Chemistry of Azapolyene and Ylide Intermediates Laboratory.

