Scientific Russia: Catch up and overtake: St Petersburg University scientists find a way to make "weak" catalysts more active
Russian scientists have compared three groups of organic catalysts to identify that the most active among organic catalyst are iodine compounds. They could also increase the efficiency of even "weaker" substances by changing the spatial environment of the atom participating in the reaction. This will help improve organic catalysts that are widely used in the synthesis of drugs and polymeric materials.
Organic catalysts are compounds that accelerate chemical reactions. They are of great interest to scientists because they are resistant to moisture, do not oxidise in air, and, unlike metal-containing analogues, are environmentally friendly and stable. This ensures that they can be removed from the reaction mixture after conversion and reused. Due to these properties, they are promising for the synthesis of a wide range of substances not only in laboratories, but also in industry.
The study is supported by a grant from the Russian Science Foundation. The research findings are published in Organic & Biomolecular Chemistry.
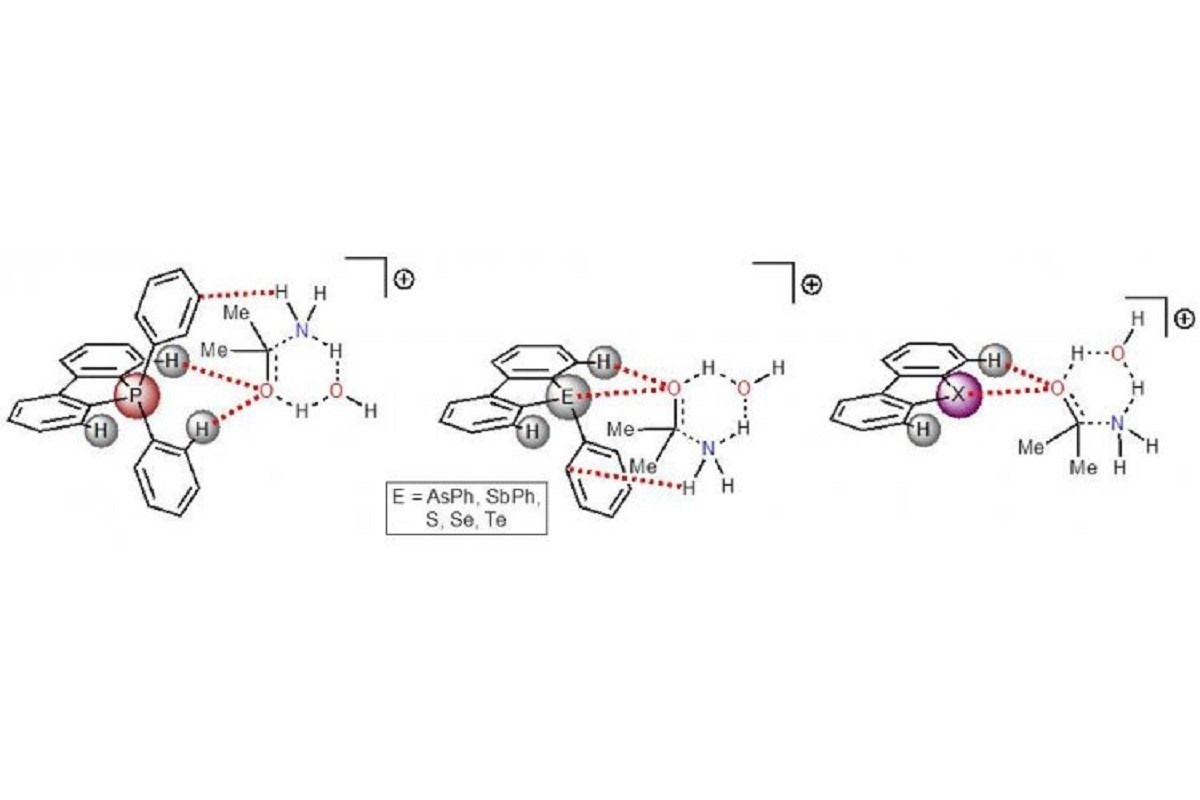
Today, the properties of organic compounds have been well studied. These properties are active as catalysts due to halogen atoms, in particular iodine, which acts as an alternative to the metal cation. Such catalysts bind to the reactant and accelerate the conversion. In addition to halogen-containing molecules, catalytic activity was found in compounds containing chalcogens, i.e. elements of the sixteenth group adjacent to the halogens of the Periodic table of Dmitri Mendeleev. These include sulphur, selenium, and tellurium. However, the variety of catalysts is not limited to this. Recently, the ability to accelerate chemical reactions was discovered in molecules containing pnictogens, i.e. elements of the fifteenth group, which includes phosphorus, arsenic, and antimony. Yet it is still unknown which of the catalysts, i.e. halogen-, chalcogen- or pnictogen-containing catalysts, work better.

Scientists at St Petersburg University, St Petersburg, compared the activity of nine types of catalysts containing halogens, chalcogens, or pnictogens. First, the authors calculated the electrostatic potential for each molecule. The electrostatic potential is the charge distributed over the surface of the molecule and allowing it to interact with other substances. Halogen compounds had the highest value, while pnictogen compounds had the lowest value. The two reactions that are most frequently encountered in the synthesis of organic compounds were used as model transformations.
The highest activity was observed for the iodine-containing catalyst, since this molecule had the highest surface charge on the central atom. However, calculations have shown that in certain cases compounds of pnictogens and chalcogens can achieve the same activity and even surpass it. To this end, the element has the right chemical environment, for example, aromatic rings of carbon atoms. In other words, simple and accessible chemical groups for attachment. This "neighbourhood" redistributes the charge on the surface of the molecule and, as a result, helps the atoms interact more efficiently with the necessary components, develop stable transition complexes, and thereby accelerate the transformation.
Over the past ten years, several generations of organic catalysts have already changed. We could for the first time compare the activity of various organocatalysts of the latest generation and identify important factors to further increase their activity.
Dmitrii Bolotin, Principal Investigator of the project, supported by a grant from the Russian Science Foundation, Doctor of Chemistry, and Professor in the Department of Physical Organic Chemistry at St Petersburg University
‘In future, we plan to obtain organic catalysts containing chiral groups, i.e. those that do not coincide with their mirror image, in order to involve them in asymmetric catalysis. This will make it possible to use them in the production of a wide range of biologically active compounds. Yet we have to solve a more fundamental problem. That is to choose the most efficient catalytic centres in order to attach chiral groups to them,’ said Dmitrii Bolotin, Principal Investigator of the project, supported by a grant from the Russian Science Foundation, Doctor of Chemistry, and Professor in the Department of Physical Organic Chemistry at St Petersburg University.

